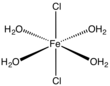
Iron(II) chloride
FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory.
Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid.
[3] Ferrous chloride is prepared by addition of iron powder to a solution of hydrochloric acid in methanol.
This reaction gives the methanol solvate of the dichloride, which upon heating in a vacuum at about 160 °C converts to anhydrous FeCl2.
Aside from use in the laboratory synthesis of iron complexes, ferrous chloride serves as a coagulation and flocculation agent in wastewater treatment, especially for wastes containing chromate or sulfides.
[13] Lawrencite, (Fe,Ni)Cl2, is the natural counterpart, and a typically (though rarely occurring) meteoritic mineral.