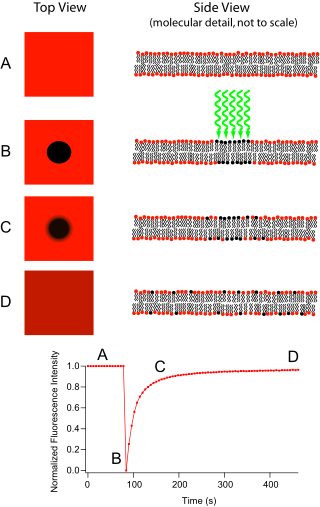
Fluorescence recovery after photobleaching
Fluorescence recovery after photobleaching (FRAP) is a method for determining the kinetics of diffusion through tissue or cells.
It is capable of quantifying the two-dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells.
This technique is very useful in biological studies of cell membrane diffusion and protein binding.
Similar, though less well known, techniques have been developed to investigate the 3-dimensional diffusion and binding of molecules inside the cell; they are also referred to as FRAP.
The basic apparatus comprises an optical microscope, a light source and some fluorescent probe.
Fluorescent emission is contingent upon absorption of a specific optical wavelength or color which restricts the choice of lamps.
Most commonly, a broad spectrum mercury or xenon source is used in conjunction with a color filter.
The fluorophores in this region receive high intensity illumination which causes their fluorescence lifetime to quickly elapse (limited to roughly 105 photons before extinction).
Now the image in the microscope is that of a uniformly fluorescent field with a noticeable dark spot.
[1][2] Originally, the FRAP technique was intended for use as a means to characterize the mobility of individual lipid molecules within a cell membrane.
[1] While providing great utility in this role, current research leans more toward investigation of artificial lipid membranes.
Supported by hydrophilic or hydrophobic substrates (to produce lipid bilayers or monolayers respectively) and incorporating membrane proteins, these biomimetic structures are potentially useful as analytical devices for determining the identity of unknown substances, understanding cellular transduction, and identifying ligand binding sites.
After the protein of interest is made fluorescent, generally by expression as a GFP fusion protein, a confocal microscope is used to photobleach and monitor a region of the cytoplasm,[3] mitotic spindle, nucleus, or another cellular structure.
[8] [9] The mean fluorescence in the region can then be plotted versus time since the photobleaching, and the resulting curve can yield kinetic coefficients, such as those for the protein's binding reactions and/or the protein's diffusion coefficient in the medium where it is being monitored.
[10] Often the only dynamics considered are diffusion and binding/unbinding interactions, however, in principle proteins can also move via flow, i.e., undergo directed motion, and this was recognized very early by Axelrod et al.[1] This could be due to flow of the cytoplasm or nucleoplasm, or transport along filaments in the cell such as microtubules by molecular motors.
Let us look at these two limits, for the common case of bleaching a GFP fusion protein in a living cell.
and diffusion-dominated recovery, the fluorescence is described by an equation derived by Soumpasis[11] (which involves modified Bessel functions
It is also assumed that the recovery can be modelled by diffusion in two dimensions, that is also both uniform and isotropic.
Note that just because the Soumpasis can be fitted adequately to data does not necessarily imply that the assumptions are true and that diffusion dominates recovery.
If a large number of proteins bind to sites in a small volume such that there the fluorescence signal is dominated by the signal from bound proteins, and if this binding is all in a single state with an off rate koff, then the fluorescence as a function of time is given by[15] Note that the recovery depends on the rate constant for unbinding, koff, only.
This more complex behavior implies that a model with several parameters is required to describe the data; models with only either a single diffusion constant D or a single off rate constant, koff, are inadequate.
[2] Unfortunately, a single FRAP curve may provide insufficient evidence to reliably and uniquely fit (possibly noisy) experimental data.
Sadegh Zadeh et al. [16] have shown that FRAP curves can be fitted by different pairs of values of the diffusion constant and the on-rate constant, or, in other words, that fits to the FRAP are not unique.
Then more data is required, e.g., by bleaching areas of different sizes,[14] determining some model parameters independently, etc.