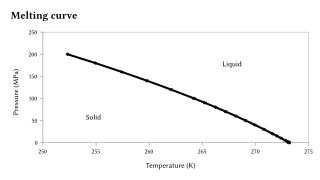
Melting point
Several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath.
The oil bath is heated (and stirred) and with the aid of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed.
the extremely high melting point (typically considered to be above, say, 1,800 °C) may be determined by heating the material in a black body furnace and measuring the black-body temperature with an optical pyrometer.
The constants in this equation are not known with sufficient accuracy, causing errors in the extrapolation to become larger at higher temperatures.
This establishes the primary calibration temperature and can be expressed in terms of current through the pyrometer lamp.
In determining melting points of a refractory substance by this method, it is necessary to either have black body conditions or to know the emissivity of the material being measured.
To form such a cavity, a hole is drilled perpendicular to the long axis at the center of a rod of the material.
These rods are then heated by passing a very large current through them, and the radiation emitted from the hole is observed with an optical pyrometer.
The point of melting is indicated by the darkening of the hole when the liquid phase appears, destroying the black body conditions.
Today, containerless laser heating techniques, combined with fast pyrometers and spectro-pyrometers, are employed to allow for precise control of the time for which the sample is kept at extreme temperatures.
Such experiments of sub-second duration address several of the challenges associated with more traditional melting point measurements made at very high temperatures, such as sample vaporization and reaction with the container.
This phenomenon is used in technical applications to avoid freezing, for instance by adding salt or ethylene glycol to water.
For example, for three structural isomers with molecular formula C5H12 the melting point increases in the series isopentane −160 °C (113 K) n-pentane −129.8 °C (143 K) and neopentane −16.4 °C (256.8 K).
[15] Likewise in xylenes and also dichlorobenzenes the melting point increases in the order meta, ortho and then para.
In highly symmetrical molecules the crystal phase is densely packed with many efficient intermolecular interactions resulting in a higher enthalpy change on melting.
An attempt to predict the bulk melting point of crystalline materials was first made in 1910 by Frederick Lindemann.
Melting initiates when the amplitude of vibration becomes large enough for adjacent atoms to partly occupy the same space.
The Lindemann criterion states that melting is expected when the vibration root mean square amplitude exceeds a threshold value.
Assuming that all atoms in a crystal vibrate with the same frequency ν, the average thermal energy can be estimated using the equipartition theorem as[18] where m is the atomic mass, ν is the frequency, u is the average vibration amplitude, kB is the Boltzmann constant, and T is the absolute temperature.
[22] The Alfa Aesar and patent data have been summarized in (respectively) random forest[21] and support vector machines.