Tubulin
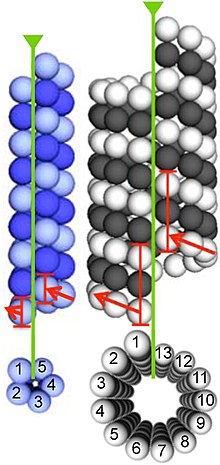
In contrast, tubulin polymers (microtubules) tend to be much bigger than actin filaments due to their cylindrical nature.
In eukaryotes, microtubules are major components of the cytoskeleton, and function in many processes, including structural support, intracellular transport, and DNA segregation.
[18] While structurally highly similar to eukaryotic tubulins, they have several unique features, including chaperone-free folding and weak dimerization.
FtsZ can polymerize into tubes, sheets, and rings in vitro, and forms dynamic filaments in vivo.
TubZ functions in segregating low copy-number plasmids during bacterial cell division.
The protein forms a structure unusual for a tubulin homolog; two helical filaments wrap around one another.
[24] In addition, several anti-worm drugs preferentially target the colchicine site of β-Tubulin in worm rather than in higher eukaryotes.
[33] Human β-tubulins subtypes include:[citation needed] γ-Tubulin, another member of the tubulin family, is important in the nucleation and polar orientation of microtubules.
It is found primarily in centrosomes and spindle pole bodies, since these are the areas of most abundant microtubule nucleation.
Besides forming a γ-TuRC to nucleate and organize microtubules, γ-tubulin can polymerize into filaments that assemble into bundles and meshworks.
[34] Human γ-tubulin subtypes include: Members of the γ-tubulin ring complex: Delta (δ) and epsilon (ε) tubulin have been found to localize at centrioles and may play a role in centriole structure and function, though neither is as well-studied as the α- and β- forms.
[37] CetZ (D4GVD7) is found in the euryarchaeal clades of Methanomicrobia and Halobacteria, where it functions in cell shape differentiation.
It protects phage DNA from host defenses like restriction enzymes and type I CRISPR-Cas systems.
[46] The anti-worm drugs mebendazole and albendazole as well as the anti-gout agent colchicine bind to tubulin and inhibit microtubule formation.
While the former ultimately lead to cell death in worms, the latter arrests neutrophil motility and decreases inflammation in humans.
These modifications include detyrosination, acetylation, polyglutamylation, polyglycylation, phosphorylation, ubiquitination, sumoylation, and palmitoylation.