Germylene
Although germylenes still have some sp2 hybridization character, the larger energy gap between s and p-orbitals for germanium permits the retainment of 4s24p2 electron configuration to some degree.
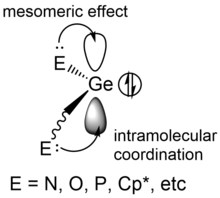
[4] Free germylenes have to be stabilized kinetically or thermodynamically due to their high reactivity originating from the vacant p-orbital.
Carbon substituted germylenes can be synthesized using various methods: (1) reduction of dibromogermanes with reducing agents like lithium naphthalene (LiNp) or potassium graphite (KC8), etc., (2) photolysis of strained cyclogermanes or Ge(IV) species, (3) substitution of a dihalo Ge(II) precursor species with nucleophiles like organometallic ligands (e.g.
Typically, the strategy for synthesizing five-membered N-heterocyclic tetrylene involves the reaction between N-substituted 1,4-diaza-1,3-butadiene, the alkali metal based reducing agents and group 14 halides.
[12] In the case of n-heterocyclic germylene (NHGe) synthesis, the method involves an initial reduction of N-substituted 1,4-diaza-1,3-butadiene by lithium.
[13] The synthetic strategy of CAAGe involves the synthesis of a α-β-unsaturated imine from a ketone and an amine via condensation followed by the treatment with GeCl2·dioxane.
[11] In 2016, Muller et al reported the synthesis of a unique homoconjugation stabilized germylene in a relatively high yield by the reaction between hafnocene dichloride and dipotassium germacyclopentadienediide in THF at -80 °C.
[15][16] The product is stabilized by a remote interaction between a C=C double bond and vacant p-orbital of Ge center through homoconjugation.
[11] The pincer based germylene is of great importance not only for their ability to stabilize transition metal species via chelation effects in homogeneous catalysis, but also for its serving as a good luminescence source.
The mechanism for insertion of free Me2Ge into the C-Br bond of benzyl bromide was reported to be a two-step, radical abstraction-recombination process under thermal and photolytical conditions.
[29] However, the interaction between halogen electrons and empty p-orbital of the germylene may result in the formation of a donor-acceptor complex before occurrence of any of the insertion mechanisms.
[5] During complexation with donors, the germylenes stay in the singlet ground state, where the lone pair is placed in the high-s-character orbital, while the heteroatom-containing donors like R2O, ROH, R2S, R3P, R3N and RCl interact with the vacant p-orbital at germanium center, which could stabilize the singlet germylene and prevent further polymerization.
[37] The absorption bands of adducts commonly exhibits shorter wavelengths in comparison to those of the free germylenes due to substituent-influenced n-p transitions at the Ge center.
[11] Oxidative addition and reductive elimination, along with the related Mn+/M(n+2)+ redox couples are of great significance to the transition metal catalysis.