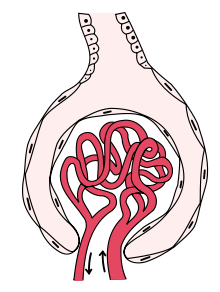
Glomerulonephritis
Secondary causes are associated with certain infections (bacterial, viral or parasitic pathogens), drugs, systemic disorders (SLE, vasculitis), or diabetes.
Although decreased intravascular oncotic (i.e. osmotic) pressure partially explains the patient's edema, more recent studies have shown that extensive sodium retention in the distal nephron (collecting duct) is the predominant cause of water retention and edema in the nephrotic syndrome.
In this syndrome, inflammatory damage to cells lining the glomerulus are thought to result in destruction of the epithelial barrier, leading to blood being found in the urine.
Minimal change disease typically presents with edema, an increase in proteins passed from urine and decrease in blood protein levels, and an increase in circulating lipids (i.e., nephrotic syndrome) and is the most common cause of the nephrotic syndrome in children.
This form of glomerulonephritis may be associated with conditions such as HIV and heroin abuse, or inherited as Alport syndrome.
The basement membrane may completely surround the granular deposits, forming a "spike and dome" pattern.
Tubules also display the symptoms of a typical Type III hypersensitivity reaction, which causes the endothelial cells to proliferate, which can be seen under a light microscope with a PAS stain.
[citation needed] In extremely rare cases, the disease has been known to run in families, usually passed down through the females.
These forms usually progress to end-stage kidney failure (ESKF) over weeks to years (depending on type).
IgA nephropathy, also known as Berger's disease, is the most common type of glomerulonephritis, and generally presents with isolated visible or occult hematuria, occasionally combined with low grade proteinuria, and rarely causes a nephritic syndrome characterised by proteinuria, and visible blood in the urine.
IgA nephropathy is classically described as a self-resolving form in young adults several days after a respiratory infection.
It typically occurs 1–4 weeks after a pharyngeal infection with this bacterium, and is likely to present with malaise, a slight fever, nausea and a mild nephritic syndrome of moderately increased blood pressure, gross haematuria, and smoky-brown urine.
[2] : 501 [6] Membranoproliferative GN (MPGN), also known as mesangiocapillary glomerulonephritis,[2]: 502 is characterised by an increase in the number of cells in the glomerulus, and alterations in the glomerular basement membrane.
Formation of crescents is initiated by passage of fibrin into the Bowman space as a result of increased permeability of glomerular basement membrane.
Rapid growing and fibrosis of crescents compresses the capillary loops and decreases the Bowman space, which leads to kidney failure within weeks or months.
[citation needed] Some forms of glomerulonephritis are diagnosed clinically, based on findings on history and examination.