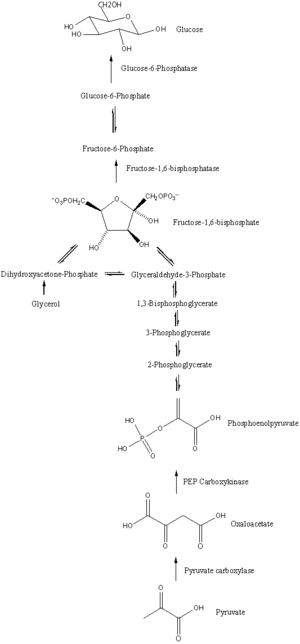
Gluconeogenesis
[2] In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc.
In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or intermediates of glycolysis (see figure).
Under conditions of prolonged fasting, acetone derived from ketone bodies can also serve as a substrate, providing a pathway from fatty acids to glucose.
[5] The gluconeogenesis pathway is highly endergonic until it is coupled to the hydrolysis of ATP or GTP, effectively making the process exergonic.
[6] In humans the main gluconeogenic precursors are lactate, glycerol (which is a part of the triglyceride molecule), alanine and glutamine.
[3][11] In nonruminants, including human beings, propionate arises from the β-oxidation of odd-chain and branched-chain fatty acids, and is a (relatively minor) substrate for gluconeogenesis.
[9] The glyoxylate shunt comprises two enzymes, malate synthase and isocitrate lyase, and is present in fungi, plants, and bacteria.
[16][17] Genes coding for malate synthase alone (but not isocitrate lyase) have been identified in other animals including arthropods, echinoderms, and even some vertebrates.
[26] In all species, the formation of oxaloacetate from pyruvate and TCA cycle intermediates is restricted to the mitochondrion, and the enzymes that convert Phosphoenolpyruvic acid (PEP) to glucose-6-phosphate are found in the cytosol.
This system of reciprocal control allow glycolysis and gluconeogenesis to inhibit each other and prevents a futile cycle of synthesizing glucose to only break it down.
The majority of the enzymes responsible for gluconeogenesis are found in the cytosol; the exceptions are mitochondrial pyruvate carboxylase and, in animals, phosphoenolpyruvate carboxykinase.
[29] The rate of gluconeogenesis is ultimately controlled by the action of a key enzyme, fructose-1,6-bisphosphatase, which is also regulated through signal transduction by cAMP and its phosphorylation.
Global control of gluconeogenesis is mediated by glucagon (released when blood glucose is low); it triggers phosphorylation of enzymes and regulatory proteins by Protein Kinase A (a cyclic AMP regulated kinase) resulting in inhibition of glycolysis and stimulation of gluconeogenesis.
[30] Insulin can no longer inhibit the gene expression of enzymes such as PEPCK which leads to increased levels of hyperglycemia in the body.
[37] However, a prebiotic glycolysis would follow the same chemical mechanisms as gluconeogenesis, due to microscopic reversibility, and in this view would have occurred at the same time.
[39] Such chemistry could have occurred in hydrothermal environments, including temperature gradients and cycling of freezing and thawing.
Mineral surfaces might have played a role in the phosphorylation of metabolic intermediates from gluconeogenesis and have to been shown to produce tetrose, hexose phosphates, and pentose from formaldehyde, glyceraldehyde, and glycolaldehyde.
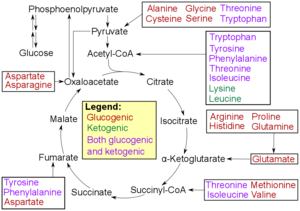
- Glucogenic amino acids have this ability
- Ketogenic amino acids do not. These products may still be used for ketogenesis or lipid synthesis .
- Some amino acids are catabolized into both glucogenic and ketogenic products.