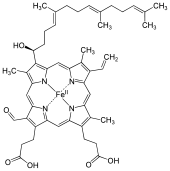
Heme A
[2] Heme A was first isolated by the German biochemist Otto Warburg in 1951 and shown by him to be the active component of the integral membrane metalloprotein cytochrome c oxidase.
In the important respiratory protein cytochrome c oxidase (CCO) this ligand 5 for the heme A at the oxygen reaction center is a histidyl group.
The iron of the heme A of cytochrome a3 is sometimes bound by 5 other atoms leaving the sixth site available to bind dioxygen (molecular oxygen).
The two heme A groups in CCO are thought to readily exchange electrons between each other, the copper ions and the closely associated protein cytochrome c. Both the formyl group and the isoprenoid side chain are thought to play important roles in conservation of the energy of oxygen reduction by cytochrome c oxidase.
CCO is thought to be responsible for conserving the energy of dioxygen reduction by pumping protons into the inter-membrane mitochondrial space.