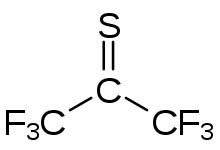
Hexafluorothioacetone
[2] Hexafluorothioacetone was first produced by Middleton in 1961 by boiling bis-(perfluoroisopropyl)mercury with sulfur.
[4] Thiols reacting with hexafluorothioacetone yield disulfides or a dithiohemiketal: With mercaptoacetic acid, instead of a thiohemiketal, water elimination yields a ring shaped molecule called a dithiolanone -CH2C(O)SC(CF3)2S- (2,2-di(trifluoromethyl)-1,3-dithiolan-4-one).
[4] Aqueous hydrogen chloride results in the formation of a dimeric disulfide CH(CF3)2SSC(CF3)2Cl.
Strong organic acids add water to yield a disulfide compound CH(CF3)2SSC(CF3)2OH.
[6] Hexafluorothioacetone is highly reactive to alkenes and dienes combining via addition reactions.