Hexanitrobenzene
Hexanitrobenzene, also known as HNB, is a nitrobenzene compound in which six nitro groups are bonded to all six positions of a central benzene ring.
It is a high-density explosive compound with chemical formula C6N6O12, obtained by oxidizing the amine group of pentanitroaniline with hydrogen peroxide in sulfuric acid.
The stable conformation of this molecule has the nitro groups rotated out of the plane of the central benzene ring.
[2] HNB has the undesirable property of being moderately sensitive to light and, therefore, hard to utilize safely.
During World War II, a method of synthesis of hexanitrobenzene was suggested in Germany, and the product was supposed to be manufactured on a semi-industrial scale according to the following scheme: Complete nitration of benzene is practically impossible because the nitro groups are deactivating groups for further nitration.

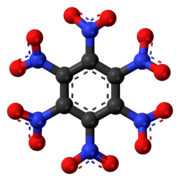

Left: Ball-and-stick structure.
Right: van der Waals space-filling structure.