Hund's rule of maximum multiplicity
The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity.
The lower energy and increased stability of the atom arise because the high-spin state has unpaired electrons of parallel spin, which must reside in different spatial orbitals according to the Pauli exclusion principle.
[3] As a result of Hund's rule, constraints are placed on the way atomic orbitals are filled in the ground state using the Aufbau principle.
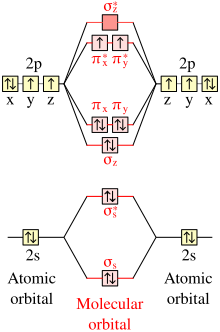
The most important example is the dioxygen molecule, O2, which has two degenerate pi antibonding molecular orbitals (π*) occupied by only two electrons.
In accordance with Hund's rule, the ground state is triplet oxygen with two unpaired electrons in singly occupied orbitals.