Transition metal hydride
Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions.
The best studied are the binary hydrides of palladium, which readily forms a limiting monohydride.
In fact, hydrogen gas diffuses through Pd windows via the intermediacy of PdH.
Because of their high lattice energy, these salts are typically not soluble in any solvents, a well known exception being K2ReH9.
The main exceptions include the late metals silver, gold, cadmium, and mercury, which form few or unstable complexes with direct M-H bonds.
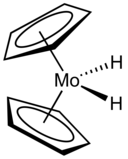
After a hiatus of several years, and following the release of German war documents on the postulated role of HCo(CO)4 in hydroformylation, several new hydrides were reported in the mid-1950s by three prominent groups in organometallic chemistry: HRe(C5H5)2 by Geoffrey Wilkinson, HMo(C5H5)(CO)3 by E. O. Fischer, and HPtCl(PEt3)2 by Joseph Chatt.
Like hydrido coordination complexes, many clusters feature terminal (bound by one M–H bond) hydride ligands.
The assignment for cluster hydrides can be challenging as illustrated by studies on Stryker's reagent [Cu6(PPh3)6H6].
The M-H bond can in principle cleave to produce a proton, hydrogen radical, or hydride.
Even if the homolytic bond strength is above that threshold the complex is still susceptible to radical reaction pathways.
This conversion results in disproportionation producing a pair of complexes with oxidation states that differ by two electrons.
Even if the hydride is not acidic enough to be deprotonated it can homolytically react with itself as discussed above for an overall one electron reduction.
As a result, kinetic studies are employed to elucidate both the relevant thermodynamic parameters.
It requires Neutron diffraction to unambiguously locate a hydride near a heavy atom crystallographically.
Non-classical hydrides have also been studied with a variety of variable temperature NMR techniques and HD Couplings.
Late transition metal hydrides characteristically show up-field shifts in their proton NMR spectra.
[19] Metal hydrides exhibit IR bands near 2000 cm−1 for νM-H, although the intensities are variable.
It was subsequently found that hydrogen gas was absorbed by mixtures of transition metal salts and Grignard reagents.
[20] The first well defined metal hydrido complex was H2Fe(CO)4, obtained by the low temperature protonation of an iron carbonyl anion.
[20] In 1957, Joseph Chatt, Bernard L. Shaw, and L. A. Duncanson described trans-PtHCl(PEt3)2 the first non-organometallic hydride (i.e., lacking a metal-carbon bond).