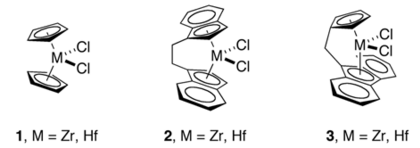
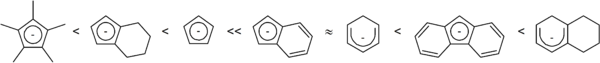Transition metal indenyl complex
18 electron indenyl complexes; however, have been shown to undergo substitution via associative pathways quite readily.
The nature of the substituents of the allyl group can strongly affect the kinetics and regiochemistry of the nucleophilic attack.
Unsurprisingly, it was found that the ease of η5 to η3 haptotropic shift correlated to the strength of the Mn-X bond.
[10] The indenyl analogues of ferrocene, which is orange, and cobaltocenium cation were first reported by Pauson and Wilkinson.
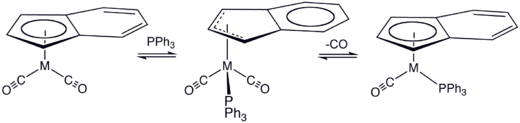
The indenyl effect was discovered by Hart-Davis and Mawby in 1969 through studies on the conversion of (η5-C9H7)Mo(CO)3CH3 to the phosphine-substituted acetyl complex, which follows bimolecular kinetics.
Hydrogenation of the arene ring in the indenyl ligand resulted in CO substitution at about half the rate of the cyclopentadienyl compound.
Shortly afterwards, Basolo tested the effect of the indene ligand on Mn(η5-C9H7)(CO)3, the cyclopentadienyl analogue of which having been shown to be inert to CO substitution.