Iodine azide
In this way, a pure solution of iodine azide results, which can then be carefully evaporated to form needle-shaped golden crystals.
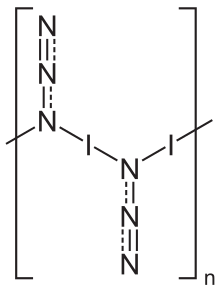
[4] In the solid state, iodine azide exists as a one-dimensional polymeric structure,[5] forming two polymorphs, both of which crystallize in an orthorhombic lattice with the space group Pbam.
[6] Iodine azide exhibits both high reactivity and comparative stability, consequences of the polarity of the I–N bond.
The N3 group introduced by substitution with iodine azide can frequently undergo subsequent reactions due to its high energy content.
Similar to bromine azide, it can add across an alkene double bond via both ionic and radical mechanisms, giving anti stereoselectivity.