Metal nitrosyl complex
The formal description of nitric oxide as NO+ does not match certain measureable and calculated properties.
In an alternative description, nitric oxide serves as a 3-electron donor, and the metal-nitrogen interaction is a triple bond.
In some complexes, however, especially when back-bonding is less important, the M-N-O angle can strongly deviate from 180°.
[6] In their framework, the factor that determines the bent vs linear NO ligands is the electron count in the metal-N-O π system.
In a further illustration, consider the {MNO} d-electron count of the [Cr(CN)5NO]3− anion.
Written in the Enemark-Feltham notation, the d electron count is {CrNO}5, and the nitrosyl is linear.
[3] Usually only of transient existence, complexes of isonitrosyl ligands are known where the NO is coordinated by its oxygen atom.
The structure of the anion can be viewed as consisting of two tetrahedra sharing an edge.
Probably relevant is the conventional self-dehydration of nitric acid: Nitric acid is used in some preparations of nitroprusside from ferrocyanide: Some anionic nitrito complexes undergo acid-induced deoxygenation to give the linear nitrosyl complex.
[2] The nitrogen atom in bent metal nitrosyls is basic, thus can be oxidized, alkylated, and protonated, e.g.: In rare cases, NO is cleaved by metal centers: Metal-nitrosyls are assumed to be intermediates in catalytic converters, which reduce the emission of NOx from internal combustion engines.
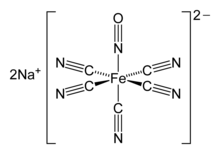
The nitroprusside anion, [Fe(CN)5NO]2−, a mixed nitrosyl cyano complex, has pharmaceutical applications as a slow release agent for NO.
The signalling function of NO is effected via its complexation to haem proteins, where it binds in the bent geometry.
Nitric oxide also attacks iron-sulfur proteins giving dinitrosyl iron complexes.