Isoprenol
It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.
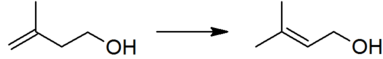
The thermodynamically preferred prenol with the more substituted double bond cannot be directly formed in the above reaction, but is produced via a subsequent isomerisation: This isomerisation reaction is catalyzed by any species which can form an allyl complex without excessive hydrogenation of the substrate, for example poisoned palladium catalysts.
[4] Isoprenol is primarily a feedstock used in the production of other more valuable chemicals.
Isoprenol and its prenol isomer are used together in the formation of ionones such as vitamin A.
[5] It is also used in the synthesis of pyrethroid pesticides , vitamin E, and citral.