Kasha's rule
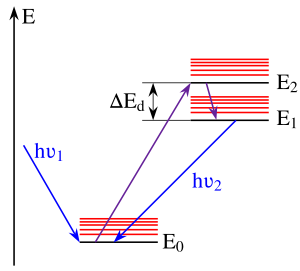
The greater the overlap, the more quickly the molecule can undergo a transition from the higher to the lower level.
Overlap between pairs is greatest when the two vibrational levels are close in energy; this tends to be the case when the vibrationless levels of the electronic states coupled by the transition (where the vibrational quantum number v is zero) are close.
However, the energy gap between S1 and S0 is greater, so here fluorescence occurs, since it is now kinetically competitive with internal conversion (IC).
[4][5] Exceptions to Kasha's rule arise when there are large energy gaps between excited states.
An example is azulene: the classical explanation is that the S1 and S2 states lie sufficiently far apart that fluorescence is observed mostly from S2.