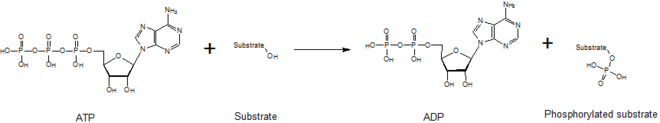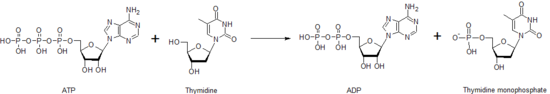Kinase
In biochemistry, a kinase (/ˈkaɪneɪs, ˈkɪneɪs, -eɪz/)[2] is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates.
Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP).
Kinases are needed to stabilize this reaction because the phosphoanhydride bond contains a high level of energy.
Kinases properly orient their substrate and the phosphoryl group within their active sites, which increases the rate of the reaction.
Alternatively, some kinases utilize bound metal cofactors in their active sites to coordinate the phosphate groups.
The addition and removal of phosphoryl groups provides the cell with a means of control because various kinases can respond to different conditions or signals.
Various other kinases act on small molecules such as lipids, carbohydrates, amino acids, and nucleotides, either for signaling or to prime them for metabolic pathways.
[15] These kinases, in conjunction with phosphatases, play a major role in protein and enzyme regulation as well as signalling in the cell.
A common point of confusion arises when thinking about the different ways a cell achieves biological regulation.
In his Hopkins Memorial Lecture, Edwin Krebs asserted that allosteric control evolved to respond to signals arising from inside the cell, whereas phosphorylation evolved to respond to signals outside of the cell.
While they are most known for their function in cell cycle control, CDKs also have roles in transcription, metabolism, and other cellular events.
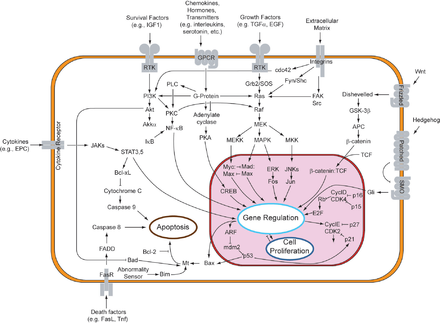
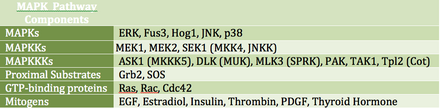
Activation of this pathway at the level of the receptor initiates a signaling cascade whereby the Ras GTPase exchanges GDP for GTP.
Its major transcriptional targets include ATF-2, Chop, c-Jun, c-Myc, DPC4, Elk-1, Ets1, Max, MEF2C, NFAT4, Sap1a, STATs, Tal, p53, CREB, and Myc.
It can also phosphorylate components in the upstream portion of the MAPK signalling cascade including Ras, Sos, and the EGF receptor itself.
It is implicated in cell processes that can lead to uncontrolled growth and subsequent tumor formation.
Mutations within this pathway alter its regulatory effects on cell differentiation, proliferation, survival, and apoptosis, all of which are implicated in various forms of cancer.
The addition of phosphate groups can change the reactivity and localization of the lipid and can be used in signal transmission.
[23] The kinase enzymes increase the rate of the reactions by making the inositol hydroxyl group more nucleophilic, often using the side chain of an amino acid residue to act as a general base and deprotonate the hydroxyl, as seen in the mechanism below.
The enzymes can also help to properly orient the ATP molecule, as well as the inositol group, to make the reaction proceed faster.
The involvement of these two kinases in cell survival, proliferation, differentiation, and inflammation makes them viable candidates for chemotherapeutic therapies.
The figure on the left shows the second phase of glycolysis, which contains two important reactions catalyzed by kinases.
This is an important step in glycolysis because it traps glucose inside the cell due to the negative charge.
[30] Other small molecules that are substrates of kinases include creatine, phosphoglycerate, riboflavin, dihydroxyacetone, shikimate, and many others.
FMN also is a precursor to flavin adenine dinucleotide(FAD), a redox cofactor used by many enzymes, including many in metabolism.
Nucleoside diphosphate kinase catalyzes production of thymidine triphosphate, dTTP, which is used in DNA synthesis.
Because of this, thymidine kinase activity is closely correlated with the cell cycle and used as a tumor marker in clinical chemistry.
[36] Patients with mutations in the thymidine kinase gene may have a certain type of mitochondrial DNA depletion syndrome, a disease that leads to death in early childhood.