Knorr quinoline synthesis
The Knorr quinoline synthesis is an intramolecular organic reaction converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid.
A reaction mechanism identified a N,O-dicationic intermediate A with excess acid capable of ring-closing and a monocationic intermediate B which fragments to aniline and (ultimately to) acetophenone.
Aniline reacts with another equivalent of benzoylacetanilide before forming the 4-hydroxyquinoline.
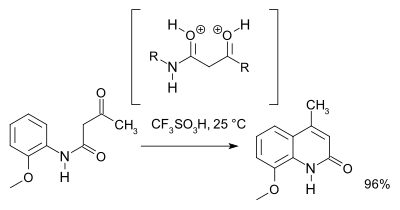
A 2007 study[3] revised the reaction mechanism showing that based on NMR spectroscopy and theoretical calculations an O,O-dicationic intermediate (a superelectrophile) is favored comparing to the N,O dicationic intermediate.
For preparative purposes triflic acid is recommended: