Lead
This is unusual; ionization energies generally fall going down a group, as an element's outer electrons become more distant from the nucleus, and more shielded by smaller orbitals.
[13] Lead's lighter carbon group congeners form stable or metastable allotropes with the tetrahedrally coordinated and covalently bonded diamond cubic structure.
In lead, the inert pair effect increases the separation between its s- and p-orbitals, and the gap cannot be overcome by the energy that would be released by extra bonds following hybridization.
[23] Lead's close-packed face-centered cubic structure and high atomic weight result in a density[24] of 11.34 g/cm3, which is greater than that of common metals such as iron (7.87 g/cm3), copper (8.93 g/cm3), and zinc (7.14 g/cm3).
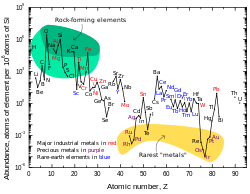
For example, the relative abundance of lead-208 can range from 52% in normal samples to 90% in thorium ores;[45] for this reason, the standard atomic weight of lead is given to only one decimal place.
[47] Lead-207 exhibits nuclear magnetic resonance, a property that has been used to study its compounds in solution and solid state,[48][49] including in the human body.
Techniques for identifying the presence of the Pb2+ ion in water generally rely on the precipitation of lead(II) chloride using dilute hydrochloric acid.
[89] In such anions, each atom is at a polyhedral vertex and contributes two electrons to each covalent bond along an edge from their sp3 hybrid orbitals, the other two being an external lone pair.
[98] Tetraethyllead, once added to automotive gasoline, was produced in larger quantities than any other organometallic compound,[93] and is still widely used in fuel for small aircraft.
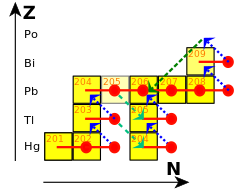
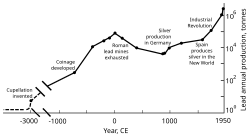
[136] Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the classical era, with an estimated annual output peaking at 80,000 tonnes.
[139] Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient Judea.
Its ease of working, its low melting point enabling the easy fabrication of completely waterproof welded joints, and its resistance to corrosion[142] ensured its widespread use in other applications, including pharmaceuticals, roofing, currency, warfare.
[160] To add to the confusion, lead bore a close relation to antimony: both elements commonly occur as sulfides (galena and stibnite), often together.
[172] Lead, in the form of Venetian ceruse, was extensively used in cosmetics by Western European aristocracy as whitened faces were regarded as a sign of modesty.
Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe.
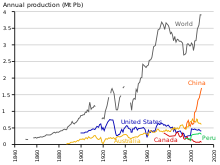
[197] According to the Metal Stocks in Society report of 2010, the total amount of lead in use, stockpiled, discarded, or dissipated into the environment, on a global basis, is 8 kg per capita.
[201] Metallic lead is further obtained from the high-lead (25–40%) slags via submerged fuel combustion or injection, reduction assisted by an electric furnace, or a combination of both.
[214] Lead metal has several useful mechanical properties, including high density, low melting point, ductility, and relative inertness.
It is inexpensive; its low melting point means small arms ammunition and shotgun pellets can be cast with minimal technical equipment; and it is denser than other common metals, which allows for better retention of velocity.
It is used as ballast in sailboat keels; its density allows it to take up a small volume and minimize water resistance, thus counterbalancing the heeling effect of wind on the sails.
[231][232] Lead is an established shielding material from radiation in nuclear science and in X-ray rooms[233] due to its denseness and high attenuation coefficient.
[268] Symptoms of lead poisoning include nephropathy, colic-like abdominal pains, and possibly weakness in the fingers, wrists, or ankles.
[citation needed] Several studies, mostly cross-sectional, found an association between increased lead exposure and decreased heart rate variability.
[293] In December 2022, Consumer Reports tested 28 dark chocolate brands and found that 23 of them contained potentially harmful levels of lead, cadmium or both.
Habitual chewing on colored plastic insulation from stripped electrical wires was found to cause elevated lead levels in a 46-year-old man.
[303] Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning,[304] continue in many parts of the world, particularly in the developing countries.
In animals, lead exhibits toxicity in many organs, damaging the nervous, renal, reproductive, hematopoietic, and cardiovascular systems after ingestion, inhalation, or skin absorption.
[315] In the United States, environmental regulations reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems.
[320] Lead may still be found in harmful quantities in stoneware,[321] vinyl[322] (such as that used for tubing and the insulation of electrical cords), and Chinese brass.
[335] Millet grass Urochloa ramosa has the ability to accumulate significant amounts of metals such as lead and zinc in its shoot and root tissues making it an important plant for remediation of contaminated soils.








Carbon
Hydrogen
Lead