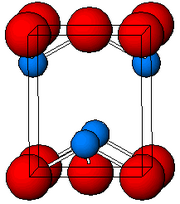
Lead(II) oxide
The pyramidal nature indicates the presence of a stereochemically active lone pair of electrons.
The PbO can be changed from massicot to litharge or vice versa by controlled heating and cooling.
Metallic lead is obtained by reducing PbO with carbon monoxide at around 1,200 °C (2,200 °F):[13] The red and yellow forms of this material are related by a small change in enthalpy: PbO is amphoteric, which means that it reacts with both acids and with bases.
Adding PbO to industrial ceramics (as well as glass) makes the materials more magnetically and electrically inert (by raising their Curie temperature) and it is often used for this purpose.
It was an unscrupulous practice in some small factories but it became rampant in China and forced many honest manufacturers to label their boxes "lead-free" after the scandal went mainstream in 2013.
In powdered tetragonal litharge form, it can be mixed with linseed oil and then boiled to create a weather-resistant sizing used in gilding.
The litharge would give the sizing a dark red color that made the gold leaf appear warm and lustrous, while the linseed oil would impart adhesion and a flat durable binding surface.