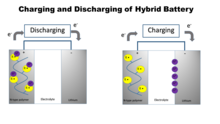
Lithium hybrid organic battery
For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4.
Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.
[1] However, amorphous oxides, in particular the V2O5 xerogel, allows lithium ions to diffuse faster and thus have a better cyclability.
However, the intercalation capacity depends on the moderate electrical conductivity and low diffusion coefficient of the lithium ions in the vanadium oxide matrix.
[6] Polyaniline eliminates the coordinated water of the V2O5 xerogel, so more lithium ions can be integrated into the structure.
[8] Re-oxidizing V(IV) to a higher oxidation state of V(V) increases initial cell voltage and specific capacity.
[9] As a result, PAni/V2O5 hybrid is a conducting network and an electroactive material in the composites, which improves electrochemical behavior.
It also prevents the irreversible structural changes made by redox cycling when the lithium ions enter the lattice.
[11] The cell was prepared by using a working electrode to assemble a half-cell configuration dry glove box with Li metal as an anode, ethyl carbonate/dimethyl carbonate as an electrophile, and a Celgard 3501 membrane as a separator.
A focus ion beam-scanning electron microscope was used to determine the morphology of the electrodes before and after the high rate pulse discharge (HRPD) cycling.
[12] After testing, pure PTMA and LiFePO4 electrode give a sharp redox peak and decrease the voltage gap between oxidation and reduction.
Furthermore, the hybrid cathodes have a lower charge-transfer resistance, allowing easier migration of Li ions through the electrode interface.