Metal carbido complex
Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis, and related catalytic industrial processes.
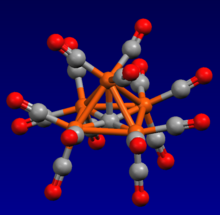
[5] Though exceptions exist, such as the nonanuclear Ruthenium cluster (μ-C)Ru9(CO)14 (μ3-η5: η2:η2-C9H7)2, containing a tripped trigonal prism geometry around the carbide.
[12] In contrast, metallocarbyne compounds are generally constitutionally heterobimetallic, with complexes containing varying coordination geometries being common.
Although, this class has also been described to some extent being analogous to the behavior of Lewis acid adduct-forming terminal nitrido and oxo complexes e.g. (PMe2Ph)2Cl-Re≡N-BCl3 and tBu(CH2)3(Br)W=O-AlBr3.
[16] Such transition metal, one coordinate-carbon bonded complexes are comparable to carbon monoxide, cyanide, and isonitrile analogues.
Synthesis of carbido clusters can be accomplished by hydrolysis, thermolysis of labile ligands, thermal rearrangements, and photolysis.
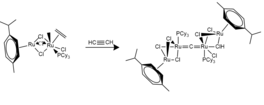
One example is the following reaction: Synthetic routes to cumulenic carbido complexes can be efficient and lead to rapid, near quantitative product formation with simple purifications.
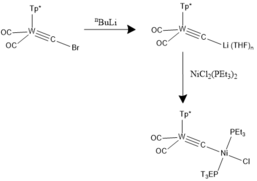
The appropriate halocarbyne precursors of choice can be reacted with organolithium reagents to afford the respective lithiocarbyne derivate by virtue of lithium/halogen exchange.
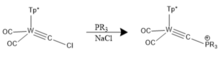
Phosphine-based analogues were first introduced by Templeton and co.[21] These types of complexes can be characterized crystallographically and are distinguishable by their Cs symmetry.
[25] Another example of a terminal carbido complex is Li[MoC(NR2)3] (Mo-C distance of 172 pm), which forms upon deprotonation of the respective methylidyne precursor.