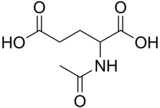
N-Acetylglutamic acid
In Rhizobium, extracellular build-up of N-acetylglutamic acid is due to metabolism involving nod factor genes on a symbiotic plasmid.
In prokaryotes with non-cyclic ornithine production, NAGS is the sole method of N-acetylglutamic acid synthesis and is inhibited by arginine.
[4] In vertebrae and mammals, N-acetylglutamic acid is the allosteric activator molecule to mitochondrial carbamyl phosphate synthetase I (CPSI) which is the first enzyme in the urea cycle.
In the liver and small intestines, N-acetylglutamic acid-dependent CPSI produces citrulline, the second intermediate in the urea cycle.
[7] In contrast, N-acetylglutamic acid is not the allosteric cofactor to carbamyl phosphate synthetase found in the cytoplasm, which is involved in pyrimidine synthesis.
Furthermore, N-acetylglutamic acid can be found in many commonly consumed foods such as soy, corn, and coffee, with cocoa powder containing a notably high concentration.
[9] Deficiency in N-acetylglutamic acid in humans is an autosomal recessive disorder that results in blockage of urea production which ultimately increases the concentration of ammonia in the blood (hyperammonemia).
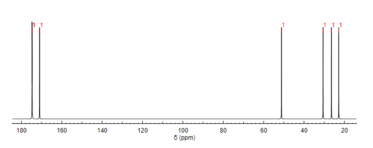
13C NMR reveals the types of carbons present in a molecule based on chemical shifts that correspond to certain functional groups.