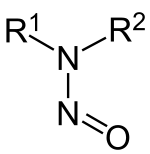
Nitrosamine
The N-N and N-O distances are 132 and 126 pm, respectively in dimethylnitrosamine,[13] one of the simplest members of a large class of N-nitrosamines Nitrosamines are not directly carcinogenic.
Metabolic activation is required to convert them to the alkylating agents that modify bases in DNA, inducing mutations.
[14][6] In 1956, two British scientists, John Barnes and Peter Magee, reported that a simple member of the large class of N-nitrosamines, dimethylnitrosamine, produced liver tumours in rats.
Subsequent studies showed that approximately 90% of the 300 nitrosamines tested were carcinogenic in a wide variety of animals.
Their presence in finished products has been tightly regulated since several food-poisoning cases in the early 20th century,[17] but consumption of large quantities of processed meats can still cause a slight elevation in gastric and oesophageal cancer risk today.
[23][24] Stomach acid catalyzes nitrosamine compound formation and is the main location of the reaction during digestion.
[25][30] Vitamin C and erythorbic acid are already commonly used in the meat industry because they enhance the binding of nitrite to myoglobin, encouraging the formation of the desired pink color.