2019 revision of the SI
In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artefacts such as the standard kilogram.
The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements.
[7]: 23 These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.
[Note 1] The kilogram remained defined by a physical prototype, leaving it the only artefact upon which the SI unit definitions depended.
Although they were designed for long-term stability, the prototype kilogram and its secondary copies have shown small variations in mass relative to each other over time; they are not thought to be adequate for the increasing accuracy demanded by science, prompting a search for a suitable replacement.
With the 2019 redefinition, the SI became wholly derivable from natural phenomena with most units being based on fundamental physical constants.
Since 1960, technological advances have made it possible to address weaknesses in the SI such as the dependence on a physical artefact to define the kilogram.
[14] In 1921 the Convention of the Metre was revised and the mandate of the CGPM was extended to provide standards for all units of measure, not just mass and length.
In the ensuing years, the CGPM took on responsibility for providing standards of electrical current (1946), luminosity (1946), temperature (1948), time (1956), and molar mass (1971).
[15] The 9th CGPM in 1948 instructed the CIPM "to make recommendations for a single practical system of units of measurement, suitable for adoption by all countries adhering to the Metre Convention".
[16] The recommendations based on this mandate were presented to the 11th CGPM (1960), where they were formally accepted and given the name "Système International d'Unités" and its abbreviation "SI".
[21] Newcastle University metrologist Peter Cumpson has since identified mercury vapour absorption or carbonaceous contamination as possible causes of this drift.
[22][23] At the 21st meeting of the CGPM (1999), national laboratories were urged to investigate ways of breaking the link between the kilogram and a specific artefact.
[25] At its 23rd meeting (2007), the CGPM mandated the CIPM to investigate the use of natural constants as the basis for all units of measure rather than the artefacts that were then in use.
[30] The CIPM, however, presented a resolution for consideration at the 24th CGPM (17–21 October 2011) to agree to the new definitions in principle, but not to implement them until the details had been finalised.
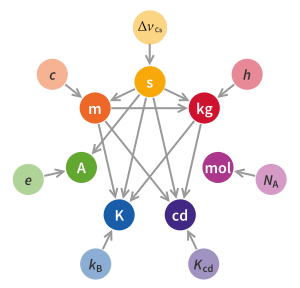
These are:[Note 5] The redefinition retains unchanged the numerical values associated with the following constants of nature: The seven SI defining constants above, expressed in terms of derived units (joule, coulomb, hertz, lumen, and watt), are rewritten below in terms of the seven base units (second, metre, kilogram, ampere, kelvin, mole, and candela);[4] the dimensionless unit steradian (symbol sr) is also used: As part of the redefinition, the International Prototype of the Kilogram was retired and definitions of the kilogram, the ampere, and the kelvin were replaced.
"[42] The kilogram may be expressed directly in terms of the defining constants: Leading to The definition of the ampere underwent a major revision.
Rather than using the triple point of water to fix the temperature scale, the new definition uses the energy equivalent as given by Boltzmann's equation.
The kelvin may be expressed directly in terms of the defining constants as: The previous definition of the mole linked it to the kilogram.
The revised definition breaks that link by making a mole a specific number of entities of the substance in question.
One of the following had to change: The wording of the 9th SI Brochure[4][Note 8] implies that the first statement remains valid, which means the second is no longer exactly true.
Appendix 2 to the 9th SI Brochure states that "the molar mass of carbon 12, M(12C), is equal to 0.012 kg⋅mol−1 within a relative standard uncertainty equal to that of the recommended value of NAh at the time this Resolution was adopted, namely 4.5×10−10, and that in the future its value will be determined experimentally",[49][50] which makes no reference to the dalton and is consistent with either statement.
For example, once length and time had been established, the universal gravitational constant G could, from a dimensional point of view, be used to define mass.
[24] On 1 September 2012 the European Association of National Metrology Institutes (EURAMET) launched a formal project to reduce the relative difference between the Kibble balance and the silicon sphere approach to measuring the kilogram from (17±5)×10−8 to within 2×10−8.
[65] Concerns that the authors of the proposal had failed to address the impact of breaking the link between the mole, kilogram, dalton, and the Avogadro constant (NA) have also been expressed.
[Note 13] This direct link has caused many to argue that the mole is not a true physical unit but, according to the Swedish philosopher Johansson, a "scaling factor".
[71] Following the proposal to redefine the mole by fixing the value of the Avogadro constant, Brian Leonard of the University of Akron, writing in Metrologia, proposed that the dalton (Da) be redefined such that NA = (g/Da) mol−1, but that the unified atomic mass unit (mu) retain its current definition based on the mass of 12C, ceasing to exactly equal the dalton.
Wojciech T. Chyla, approaching the structure of SI from a philosophical point of view in the Journal of the Polish Physical Society, argued that temperature is not a real base unit but is an average of the thermal energies of the individual particles that comprise the body concerned.