n-Octyl β-D-thioglucopyranoside
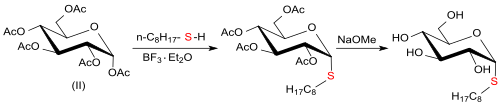
The synthesis of n-octyl-β-d-thioglucopyranoside[4] starts from D-glucose (I) which is prepared with acetic anhydride and concentrated sulfuric acid to give α-d-glucopyranose pentaacetate (pentaacetylglucose) (II).
[7] The reaction of d-glucose with 1-octanethiol and Olah's reagent[8] (70% hydrogen fluoride HF in pyridine) produces an anomeric mixture of n-octyl-1-thio-α,β-d-glucopyranoside in 95% yield which contains 44% α-anomeres and 56% β-anomers.
Compared with the O-glucoside n-octyl-β-d-glucopyranoside, which has already been introduced earlier as detergent for biochemical applications, the analogous S-glucoside OTG appears to be particularly suitable due to its higher stability, especially against degradation by β-glucosidases.
For this, the solution of the solubilized protein is subject to dialysis or ion exchange chromatography in the presence of phospholipids or membrane lipid mixtures to remove the surfactant.
[2] Octylthioglucoside (15 mM) is clearly superior to its O-analog octyl glucoside (OT) in the solubilization and stabilization against thermal and light-induced denaturation of the light-driven proton pump Bacteriorhodopsin from the biomembranes of halobacteria.