Trimethylplatinum iodide
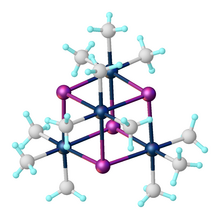
[2] The complex exists as a tetramer: a cubane-type cluster with four octahedral Pt(IV) centers linked by four iodides as triply bridging ligands.
[3] Due to its stability, it is often utilized as a precursor en route to the synthesis of other organoplatinum compound, such as hydrosilylation catalysts.
The fluoride, bromide, chloride, and pseudohalide (OH, N3, SCN, SMe) analogues [(CH3)3PtX)]4 also exist as tetramers, all forming cubane clusters.
[1] When exposed to UV light in solution, the tetramer can undergo photolysis of two methyl radicals by reductive elimination, forming the brief Pt(II)(CH3)I species.
The cubane structure can undergo decomposition by ligand substitution and breaking of the bridging iodine, to form a variety of octahedral organoplatinum complexes.