pH-sensitive polymers
Materials may swell, collapse, or change depending on the pH of their environment.
This behavior is exhibited due to the presence of certain functional groups in the polymer chain.
The general form of the polymer is a backbone with functional "pendant groups" that hang off of it.
[2] Examples include polymethyl methacrylate polymers (pharmacologyonline 1 (2011)152-164) and cellulose acetate phthalate.
It is often evaluated as a calcium-salt for drug delivery applications(International journal of biological macromolecules 75 (2015) 409-17) .
Natural polymers have appeal because they display good biocompatibility, which makes them useful for biomedical applications.
[1] pH sensitive polymers have been created with linear block copolymer, star, branched, dendrimer, brush, and comb architectures.
However, star and branched polymers can form rod or worm-shaped micelles rather than the typical spheres.
For example, polyacids release protons to become negatively charged at high pH.
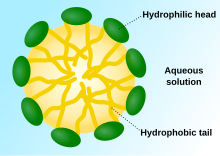
[citation needed] Polymers can also form micelles (spheres) in response to a change in pH.
[4] Additionally, a change in pH could cause micelles to swap their inner and outer molecules depending on the properties of the polymers involved.
Functional groups may need to be protected so that they do not react depending on the type of polymerization.
[1] Graft copolymers are a popular type to synthesize because their structure is a backbone with branches.
[1] Several methods can be used to measure the contact angle of a water drop on the surface of a polymer.
Researchers aim to design molecules that transition at a pH that matters for the given application.