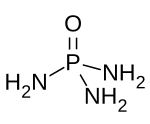
Phosphoramide
Phosphoramide is a chemical compound with the molecular formula O=P(NH2)3.
In bulk, the compound is a white solid which is soluble in polar solvents.
Phosphoramide arises from the reaction of phosphoryl chloride with ammonia.
In moist air, it hydrolyzes to an ammonium salt: It reacts with sodium hydroxide with loss of ammonia:[2] The related thiophosphoryl triamide compound S=P(NH2)3 was made from the reaction of thiophosphoryl chloride with ammonia.
[3] An example compound is the polar solvent hexamethylphosphoramide (HMPA).