Photooxygenation
[1][2] Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells.
[4] Photooxygenation reactions are initiated by a photosensitizer, which is a molecule that enters an excited state when exposed to light of a specific wavelength (e.g. dyes and pigments).
Clear distinctions can be made based on three attributes: oxidation, the involvement of light, and the incorporation of molecular oxygen into the products: Sensitizers (denoted "Sens") are compounds, such as fluorescein dyes, methylene blue, and polycyclic aromatic hydrocarbons, which are able to absorb electromagnetic radiation (usually in the visible range of the spectrum) and eventually transfer that energy to molecular oxygen or the substrate of photooxygenation process.
Many sensitizers, both naturally occurring and synthetic, rely on extensive aromatic systems to absorb light in the visible spectrum.
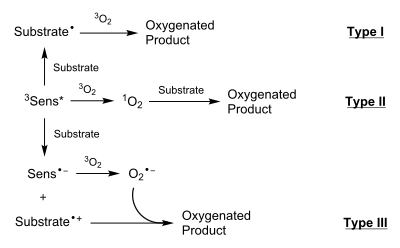
[4] The three types of photooxygenation reactions are distinguished by the mechanisms that they proceed through, as they are capable of yielding different or similar products depending on environmental conditions.
Note that the reaction proceeds through an indolizine radical cation intermediate that has not been isolated (and thus is not depicted):[9] All 3 types of photooxygenation have been applied in the context of organic synthesis.
In particular, type II photooxygenations have proven to be the most widely used (due to the low amount of energy required to generate singlet oxygen) and have been described as "one of the most powerful methods for the photochemical oxyfunctionalization of organic compounds.
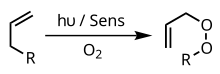
Many of the applications of type II photooxygenations in organic synthesis come from Waldemar Adam's investigations into the ene-reaction of singlet oxygen with acyclic alkenes.
[10] Through the cis effect and the presence of appropriate steering groups the reaction can even provide high regioselectively and diastereoselectivity - two valuable stereochemical controls.
Since the maximum penetration of tissues is achieved around wavelengths of 800 nm, selecting Sens that absorb around this range is advantageous as it allows for PDT to be affective on tumors beneath the outer most layer of the dermis.