Pioneer factor
They were first discovered in 2002 as factors capable of binding to target sites on nucleosomal DNA in compacted chromatin and endowing competency for gene activity during hepatogenesis.
Pioneer factors can also actively affect transcription by directly opening up condensed chromatin in an ATP-independent process.
The similarity to histone H1 explains how fork head factors are able to bind chromatin by interacting with the major groove of only the one available side of DNA wrapped around a nucleosome.
[6] NF-YA's unique DNA-binding mode and NF-YB/NF-YC's nucleosome-like properties of non-specific DNA binding impose sufficient spatial constraints to induce flanking nucleosomes to slide outward, making nearby recognition sites for other transcription factors accessible.
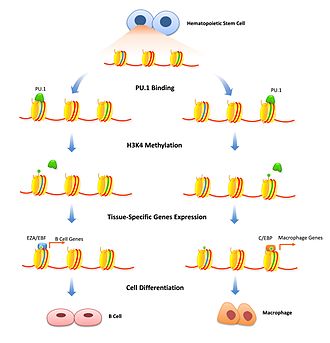
[17] SOX9 recruits histone modification enzymes MLL3 and MLL4 to deposit H3K4me1 prior to the opening of enhancers in developing hair follicle and basal cell carcinoma.
Many eukaryotic cells have CpG islands in their promoters that can be modified by methylation having adverse effects on their ability to control transcription.
[19] This phenomenon is also present in promoters without CpG islands where single cytosine residues are protected from methylation until further cell differentiation.
An example is FoxD3 preventing methylation of a cytosine residue in Alb1 enhancer, acting as a place holder for FoxA1 later in hepatic [20] as well as in CpG islands of genes in chronic lymphocytic leukemia.
These large domains are scaffolds for further protein interactions and also modify the chromatin for other pioneer factors such as FoxA1 which has been shown to bind to Grg3.
[23] Transcription factors with zinc finger DNA binding domains, such as the GATA family and glucocorticoid receptor.
[24] The ability of pioneer factors to respond to extracellular signals to differentiate cell type has been studied as a potential component of hormone-dependent cancers.
Hormones such as estrogen and IGFI are shown to increase pioneer factor concentration leading to a change in transcription.
FoxA1 is necessary for estrogen and androgen mediated hepatocarcinogenesis and is a defining gene for ER+ luminal breast cancer, as is another pioneer factor GATA3.
High expression of pioneer factors is associated with poor prognosis with the exception of breast cancer where FoxA1 is associated with a stronger outcome.