Properties of water
Water (H2O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue.
Water is amphoteric, meaning that it can exhibit properties of an acid or a base, depending on the pH of the solution that it is in; it readily produces both H+ and OH− ions.
Under standard conditions, water is primarily a liquid, unlike other analogous hydrides of the oxygen family, which are generally gaseous.
The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2 × 10−13 seconds).
Within the Earth's atmosphere and surface, the liquid phase is the most common and is the form that is generally denoted by the word "water".
The breaking of hydrogen bonds on melting with increasing temperature in the range 0–4 °C allows for a denser molecular packing in which some of the lattice cavities are filled by water molecules.
[36] Furthermore, given that water is a good thermal insulator (due to its heat capacity), some frozen lakes might not completely thaw in summer.
This ice floats on the surface, and the salt that is "frozen out" adds to the salinity and density of the seawater just below it, in a process known as brine rejection.
Vapor pressure above 100% relative humidity is called supersaturated and can occur if the air is rapidly cooled, for example, by rising suddenly in an updraft.
The low compressibility of water means that even in the deep oceans at 4 kilometres (2.5 mi) depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.
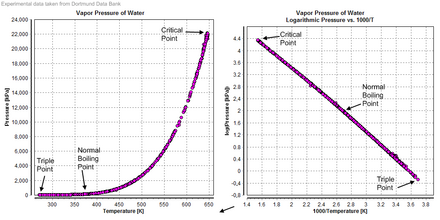
[46] Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century.
[56] This figure agrees well with what is typically seen on reverse osmosis, ultra-filtered and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants.
A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.
[56] Water can also be electrolyzed into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted.
The lone pairs are closer to the oxygen atom than the electrons sigma bonded to the hydrogens, so they require more space.
These properties include its relatively high melting and boiling point temperatures: more energy is required to break the hydrogen bonds between water molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl− anions, each being surrounded by water molecules.
The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
At that time it was known that there are motions which destroy and regenerate the weak hydrogen bond by internal rotations of the substituent water monomers.
Unlike previously reported tunneling motions in water, this involved the concerted breaking of two hydrogen bonds.
This view is based upon neutron scattering studies and computer simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in ice structures.
In 2004, a controversial paper from Stockholm University suggested that water molecules in the liquid state typically bind not to four but only two others; thus forming chains and rings.
These observations were based upon X-ray absorption spectroscopy that probed the local environment of individual oxygen atoms.
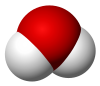
[73] The repulsive effects of the two lone pairs on the oxygen atom cause water to have a bent, not linear, molecular structure,[74] allowing it to be polar.
[77] If high amounts of nitrogen and sulfur oxides are present in the air, they too will dissolve into the cloud and raindrops, producing acid rain.
Tritium is radioactive, decaying with a half-life of 4500 days; THO exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere.
Consumption of pure isolated D2O may affect biochemical processes—ingestion of large amounts impairs kidney and central nervous system function.
Small quantities can be consumed without any ill-effects; humans are generally unaware of taste differences,[80] but sometimes report a burning sensation[81] or sweet flavor.
[98] The first decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by English chemist William Nicholson and Anthony Carlisle.
Notably, the Kelvin, Celsius, Rankine, and Fahrenheit scales were, or currently are, defined by the freezing and boiling points of water.