Ring-opening polymerization
Note: If monomer is polycyclic, the opening of a single ring is sufficient to classify the reaction as ring-opening polymerization.
Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain.
Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative.
[10][11] Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization.
Even highly strained cycloalkane rings, such as cyclopropane[12] and cyclobutane[13] derivatives, can undergo ROP.
Synthesis of polypeptides which has the oldest history of ROP, dates back to the work in 1906 by Leuchs.
[15][16] The first high-molecular weight polymers (Mn up to 105) with a repeating unit were prepared by ROP as early as in 1976.
[19] Additionally, radical ROP is useful in producing polymers with functional groups incorporated in the backbone chain that cannot otherwise be synthesized via conventional chain-growth polymerization of vinyl monomers.
For instance, radical ROP can produce polymers with ethers, esters, amides, and carbonates as functional groups along the main chain.
[19][20] Anionic ring-opening polymerizations (AROP) involve nucleophilic reagents as initiators.
Monomers with a three-member ring structure - such as epoxides, aziridines, and episulfides - undergo anionic ROP.
[20] A typical example of anionic ROP is that of ε-caprolactone, initiated by an alkoxide.
Examples of cyclic monomers that polymerize through this mechanism include lactones, lactams, amines, and ethers.
[19] The mechanism is affected by the stability of the resulting cationic species.
For example, if the atom bearing the positive charge is stabilized by electron-donating groups, polymerization will proceed by the SN1 mechanism.
[20] The cationic species is a heteroatom and the chain grows by the addition of cyclic monomers thereby opening the ring system.
[19] CROP can be a living polymerization and can be terminated by nucleophilic reagents such as phenoxy anions, phosphines, or polyanions.
[19] When the amount of monomers becomes depleted, termination can occur intra or intermolecularly.
The active end can "backbite" the chain, forming a macrocycle.
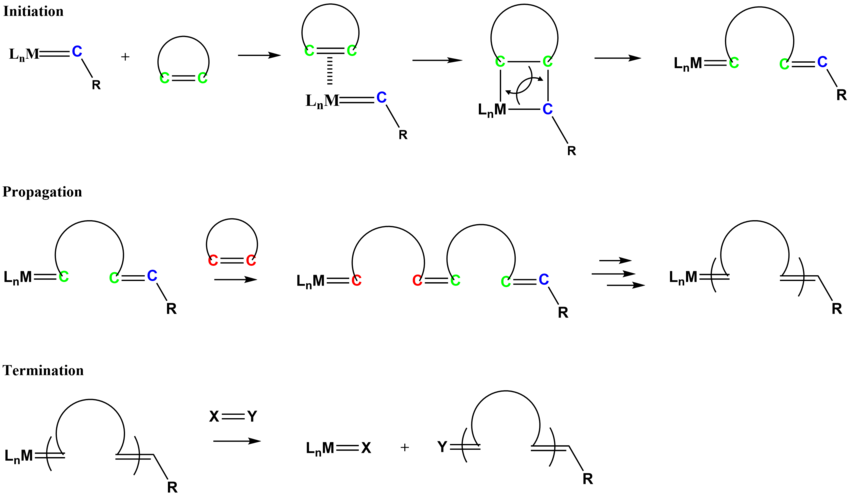
Ring-opening metathesis polymerisation (ROMP) produces unsaturated polymers from cycloalkenes or bicycloalkenes.
[19] The mechanism for ROMP follows similar pathways as olefin metathesis.
The initiation process involves the coordination of the cycloalkene monomer to the metal alkylidene complex, followed by a [2+2] type cycloaddition to form the metallacyclobutane intermediate that cycloreverts to form a new alkylidene species.
[23][24] Commercially relevant unsaturated polymers synthesized by ROMP include polynorbornene, polycyclooctene, and polycyclopentadiene.
[25] The formal thermodynamic criterion of a given monomer polymerizability is related to a sign of the free enthalpy (Gibbs free energy) of polymerization:
where: The free enthalpy of polymerization (ΔGp) may be expressed as a sum of standard enthalpy of polymerization (ΔGp°) and a term related to instantaneous monomer molecules and growing macromolecules concentrations:
Eventually, at or above the so-called ceiling temperature (Tc), at which [M]eq = [M]0, formation of the high polymer does not occur.
For example, tetrahydrofuran (THF) cannot be polymerized above Tc = 84 °C, nor cyclo-octasulfur (S8) below Tf = 159 °C.
The polymerization of a majority of monomers is accompanied by an entropy decrease, due mostly to the loss in the translational degrees of freedom.
In this situation, polymerization is thermodynamically allowed only when the enthalpic contribution into ΔGp prevails (thus, when ΔHp° < 0 and ΔSp° < 0, the inequality |ΔHp| > −TΔSp is required).
Therefore, the higher the ring strain, the lower the resulting monomer concentration at equilibrium.