Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments.
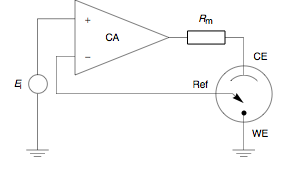
The heart of the different potentiostatic electronic circuits is an operational amplifier (op amp).
[5] It consists of an electric circuit which is usually described in terms of simple op amps.
This equipment is fundamental to modern electrochemical studies using three electrode systems for investigations of reaction mechanisms related to redox chemistry and other chemical phenomena.
In a bulk electrolysis total coulombs passed (total electric charge) is plotted against time in seconds even though the experiment measures electric current (amperes) over time.
Modern potentiostats are designed to interface with a personal computer and operate through a dedicated software package.
The automated software allows the user rapidly to shift between experiments and experimental conditions.
The computer allows data to be stored and analyzed more effectively, rapidly, and accurately than the earlier standalone devices.
It comprises an electric circuit which controls the potential across the cell by sensing changes in its resistance, varying accordingly the current supplied to the system: a higher resistance will result in a decreased current, while a lower resistance will result in an increased current, in order to keep the voltage constant as described by Ohm's law.
As a result, the variable system resistance and the controlled current are inversely proportional Since 1942, when the English electrochemist Archie Hickling (University of Leicester) built the first three electrode potentiostat,[6] substantial progress has been made to improve the instrument.
It adjusts its output to automatically control the cell current so that a condition of equilibrium is satisfied.
Prior to observing the following equations, one may note that, from an electrical point of view, the electrochemical cell and the current measurement resistor
At this point the assumption may be made that a negligible amount of current is flowing through the reference electrode.
This correlates to physical phenomenon since the reference electrode is connected to a high impedance electrometer.
is the fraction of the output voltage of the control amplifier returned to its negative input; namely the feedback factor: Combining Eqs.
Replacing the CA, a control algorithm can maintain a constant voltage
The algorithm requires software-controllable hardware such as a digital multimeter, a power supply, and a double-pole double-throw relay.
In electrochemical experiments the electrodes are the pieces of equipment that comes in immediate contact with the analyte.