Proximity labeling
Proximity labeling has been used for identifying the components of novel cellular structures and for determining protein-protein interaction partners, among other applications.
[3] DamID is a method developed in 2000 by Steven Henikoff for identifying parts of the genome proximal to a chromatin protein of interest.
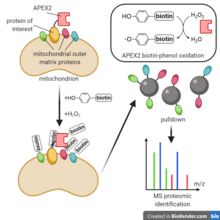
[9][10] Proximity labeling methods have been used to study the proteomes of biological structures that are otherwise difficult to isolate purely and completely, such as cilia,[11] mitochondria,[6] postsynaptic clefts,[2] p-bodies, stress granules,[12] and lipid droplets.
[13] Fusion of APEX2 with G-protein coupled receptors (GPCRs) allows for both tracking GPCR signaling at a 20-second temporal resolution[14] and also identification of unknown GPCR-linked proteins.
[19] BioID-based proximity labeling has been used to identify the molecular composition of breast cancer cell invadopodia, which are important for metastasis.
[23] This photocatalytic technology leverages the photonic energy of iridium-based photocatalysts to activate diazirine probes that can tag proximal proteins within a tight radius of about four nanometers.