Pterin
[4] The pteridine ring system contains four nitrogen atoms, reducing its aromaticity to the point that it can be attacked by nucleophile.
[5] Pterin rings are either salvaged from existing ones or produced de novo in living organisms.
Folate-dependent biosynthetic reactions include the transfer of methyl groups from 5-methyltetrahydrofolate to homocysteine to form L-methionine, and the transfer of formyl groups from 10-formyltetrahydrofolate to L-methionine to form N-formylmethionine in initiator tRNAs.
[6] It binds molybdenum to yield redox cofactors involved in biological hydroxylations, reduction of nitrate, and respiratory oxidation.
It occurs in four steps:[10] Tetrahydrobiopterin, the major unconjugated pterin in vertebrates, is involved in three families of enzymes that effect hydroxylation.
Tetrahydrobiopterin is also required for the functioning of alkylglycerol monooxygenase, whereby monoalkylglycerols are broken down to glycerol and an aldehyde.
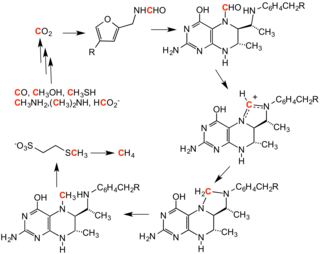
[12] Tetrahydromethanopterin is a cofactor in methanogenesis, which is a metabolism adopted by many organisms, as a form of anaerobic respiration.