Pyruvate dehydrogenase
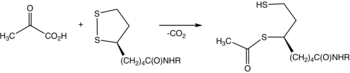
[2] In terms of details, biochemical and structural data for E1 revealed a mechanism of activation of TPP coenzyme by forming the conserved hydrogen bond with glutamate residue (Glu59 in human E1) and by imposing a V-conformation that brings the N4’ atom of the aminopyrimidine to intramolecular hydrogen bonding with the thiazolium C2 atom.
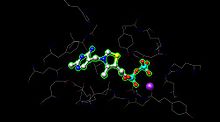
[1] The active site for pyruvate dehydrogenase (image created from PDB: 1NI4) holds TPP through metal ligation to a magnesium ion (purple sphere) and through hydrogen bonding to amino acids.
PDK is inhibited by dichloroacetic acid and pyruvate, resulting in a higher quantity of active, unphosphorylated PDH.
[4] Phosphorylation is reversed by pyruvate dehydrogenase phosphatase, which is stimulated by insulin, PEP, and AMP, but competitively inhibited by ATP, NADH, and Acetyl-CoA.
Pyruvate dehydrogenase is targeted by an autoantigen known as anti-mitochondrial antibodies (AMA), which results in progressive destruction of the small bile ducts of the liver, leading to primary biliary cirrhosis.
Some of these inflammatory responses could be related to gluten sensitivity as over 50% of the acute liver failure patients in one study exhibited a nonmitochondrial autoantibody against tissue transglutaminase.
Malfunction of the citric acid cycle due to PDH deficiency deprives the body of energy and leads to an abnormal buildup of lactate.
PDH deficiency is a common cause of lactic acidosis in newborns and often presents with severe lethargy, poor feeding, tachypnea, and cases of death have occurred.