Tissue transglutaminase
[6] tTG is the autoantigen in celiac disease, a lifelong illness in which the consumption of dietary gluten causes a pathological immune response resulting in the inflammation of the small intestine and subsequent villous atrophy.
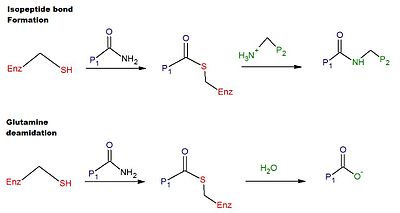
TG2 is a multifunctional enzyme that belongs to transglutaminases which catalyze the crosslinking of proteins by epsilon-(gamma-glutamyl)lysine isopeptide bonds.
[15] Similarly to other transglutaminases, tTG consists of a GTP/ GDP binding site, a catalytic domain, two beta barrel and a beta-sandwich.
[6] The thiol group attacks the carboxamide of a glutamine residue on the surface of a protein or peptide substrate, releasing ammonia, and producing a thioester intermediate.
Alternatively, the thioester intermediate can be hydrolyzed, resulting in the net conversion of the glutamine residue to glutamic acid (i.e.
[6] The deamidation of glutamine residues catalyzed by tTG is thought to be linked to the pathological immune response to gluten in celiac disease.
Once synthesized, most of the protein is found in the cytoplasm, plasma membrane and ECM, but a small fraction is translocated to the nucleus, where it participates in the control of its own expression through the regulation of transcription factors.
Mutations to these binding sites causing lower calcium affinity, decrease the enzyme's transglutaminase activity.
[23] Therefore, intracellular tTG is mostly inactive due to the relatively high concentration of GTP/GDP and the low levels of calcium inside the cell.
[20] The presence of calcium protects against the formation of both disulfide bonds, thus making the enzyme more resistant to oxidation.
(see Figure 3)[23][25][26] In the extracellular matrix, TG2 is "turned off", due primarily to the oxidizing activity of endoplasmic reticulum protein 57 (ERp57).
Among its many supposed functions, it appears to play a role in wound healing, apoptosis, and extracellular matrix development[11] as well as differentiation and cell adhesion.
[27] tTG is thought to be involved in the regulation of the cytoskeleton by crosslinking various cytoskeletal proteins including myosin, actin, and spectrin.
It is also believed that tTG may stabilize the structure of the dying cells during apoptosis by polymerizing the components of the cytoskeleton, therefore preventing the leakage of the cellular contents into the extracellular space.
[7] tTG also has GTPase activity:[5] In the presence of GTP, it suggested to function as a G protein participating in signaling processes.
[35] Anti-transglutaminase antibodies result in a form of gluten sensitivity in which a cellular response to Triticeae glutens that are crosslinked to tTG are able to stimulate transglutaminase specific B-cell responses that eventually result in the production of anti-transglutaminase antibodies IgA and IgG.
[36][37] tTG specifically deamidates the glutamine residues creating epitopes that increase the binding affinity of the gluten peptide to the antigen presenting T cells, initiating an adaptive immune response.
tTG is believed to contribute to several neurodegenerative disorders including Alzheimer, Parkinson and Huntington diseases by affecting transcription, differentiation and migration and adhesion .
Specifically, in kidney fibrosis, tTG contributes to the stabilization and accumulation of the ECM affecting TGF beta activity.
Recent studies indicate that non-enzymatic interactions play physiological roles and enable diverse TG2 functions in a context-specific manner.