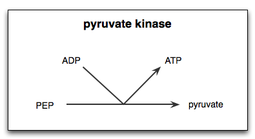
Pyruvate kinase
[2] Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.
The R-state, characterized by high substrate affinity, serves as the activated form of pyruvate kinase and is stabilized by PEP and fructose 1,6-bisphosphate (FBP), promoting the glycolytic pathway.
Phosphoenolpyruvate may have been present abiotically, and has been shown to be produced in high yield in a primitive triose glycolysis pathway.
In glycolysis, the rate-limiting steps are coupled to either the hydrolysis of ATP or the phosphorylation of ADP, causing the pathway to be energetically favorable and essentially irreversible in cells.
This final step is highly regulated and deliberately irreversible because pyruvate is a crucial intermediate building block for further metabolic pathways.
[17] Once the gluconeogenesis pathway is complete, the glucose produced is expelled from the liver, providing energy for the vital tissues in the fasting state.
Under wild-type conditions, all three of these reactions are irreversible, have a large negative free energy and are responsible for the regulation of this pathway.
[17] Pyruvate kinase activity is most broadly regulated by allosteric effectors, covalent modifiers and hormonal control.
This regulation system is responsible for the avoidance of a futile cycle through the prevention of simultaneous activation of pyruvate kinase and enzymes that catalyze gluconeogenesis.
As a result, the inhibition of pyruvate kinase by glucagon, cyclic AMP and epinephrine, not only shuts down glycolysis, but also stimulates gluconeogenesis.
Alternatively, insulin interferes with the effect of glucagon, cyclic AMP and epinephrine, causing pyruvate kinase to function normally and gluconeogenesis to be shut down.
Furthermore, glucose was found to inhibit and disrupt gluconeogenesis, leaving pyruvate kinase activity and glycolysis unaffected.
Overall, the interaction between hormones plays a key role in the functioning and regulation of glycolysis and gluconeogenesis in the cell.
Although metformin does not directly affect pyruvate kinase activity, it causes a decrease in the concentration of ATP.
[27] Heterogenous ribonucleotide proteins (hnRNPs) can act on the PKM gene to regulate expression of M1 and M2 isoforms.
[30] A glucose-sensing module contains domains that are targets for regulatory phosphorylation based on the concentrations of glucose and cAMP, which then control its import into the nucleus.
[31] Therefore, high glucose and low cAMP causes dephosphorylation of ChREBP, which then upregulates expression of pyruvate kinase in the liver.
In this manner, the harmful effects of ROS are increased and cause greater oxidative stress on the lung cells, leading to potential tumor formation.
A study of PKM2 in babies with the genetic brain disease phenylketonurics (PKU), showed elevated levels of phenylalanine and decreased effectiveness of PKM2.
This inhibitory mechanism provides insight into the role of pyruvate kinase in brain cell damage.
The low activity dimer allows for build-up of phosphoenol pyruvate (PEP), leaving large concentrations of glycolytic intermediates for synthesis of biomolecules that will eventually be used by cancer cells.
[8] Phosphorylation of PKM2 by Mitogen-activated protein kinase 1 (ERK2) causes conformational changes that allow PKM2 to enter the nucleus and regulate glycolytic gene expression required for tumor development.