Rydberg matter
[2][3] It has been formed from various elements like caesium,[4] potassium,[5] hydrogen[6][7] and nitrogen;[8] studies have been conducted on theoretical possibilities like sodium, beryllium, magnesium and calcium.
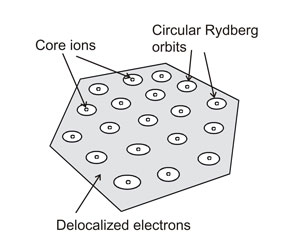
[13][14][15] Rydberg matter consists of usually[17] hexagonal[18][16] planar[19] clusters; these cannot be very big because of the retardation effect caused by the finite velocity of the speed of light.
Though Rydberg matter can be studied in the laboratory by laser probing,[20] the largest cluster reported consists of only 91 atoms,[7] but it has been shown to be behind extended clouds in space[10][21] and the upper atmosphere of planets.
[3] The way in which the electrons delocalise is to form standing waves on loops surrounding nuclei, creating quantised angular momentum and the defining characteristics of Rydberg matter.
A key variable in determining these properties is the principal quantum number n that can be any integer greater than 1; the highest values reported for it are around 100.
[25] The overview[33] provides information on Rydberg matter and possible applications in developing clean energy, catalysts, researching space phenomena, and usage in sensors.