Scholl reaction
In 1910 Scholl reported the synthesis of a quinone [3] and of perylene from naphthalene[4] both with aluminum chloride.
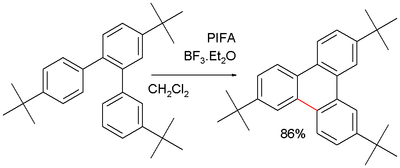
[7] Given the high reaction temperature and the requirement for strongly acidic catalysts the chemical yield often is low and the method is not a popular one.
A recurring problem is oligomerization of the product which can be prevented by blocking tert-butyl substituents:[7]
Just as in electrophilic aromatic substitution, Activating groups such as methoxy improve yield and selectivity:[7] Indeed, oxidative coupling of phenols is a research strategy in modern organic synthesis.
Reactions taking place at room-temperature with well-known one-electron oxidizing agents likely proceed through a radical cation mechanism and reactions requiring elevated temperatures likely proceed through an arenium ion mechanism.