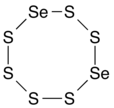
Selenium hexasulfide
Its molecular structure is an 8-membered ring, consisting of two selenium and six sulfur atoms (diselenacyclooctasulfane), analogous to the S8 ring, an allotrope of sulfur (cyclooctasulfur or cyclooctasulfane), and other 8-membered rings of selenium sulfides with formula SenS8−n.
[2] There are several isomers depending on the relative placement of the selenium atoms in the ring: 1,2-diselenacyclooctasulfane (with the two Se atoms adjacent), 1,3-diselenacyclooctasulfane, 1,4-diselenacyclooctasulfane, and 1,5-diselenacyclooctasulfane (with the Se atoms opposite).
The 1,2 isomer can be prepared by reaction of chlorosulfanes and dichlorodiselane with potassium iodide in carbon disulfide.
The reaction produces also cyclooctaselenium Se8 and all other eight-member cyclic selenium sulfides, except selenacyclooctasulfane SeS7, and several six- and seven-membered rings.
This inorganic compound–related article is a stub.