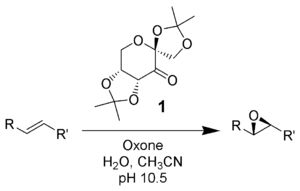
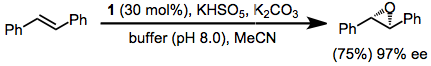
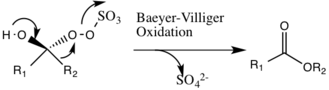
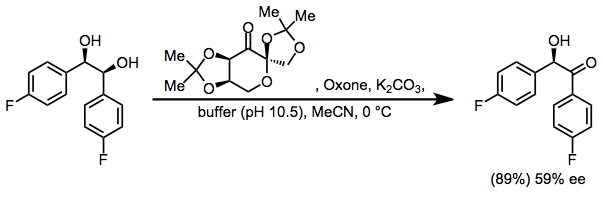
Shi epoxidation
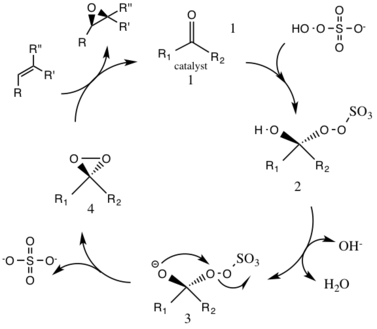
This reaction is thought to proceed via a dioxirane intermediate, generated from the catalyst ketone by oxone (potassium peroxymonosulfate).
[3][4] The reaction was first reported by Yian Shi (史一安, pinyin: Shǐ Yī-ān) is derived from D-fructose and has a stereogenic center close to the reacting center (ketone)- the rigid six-membered ring structure of the catalyst and adjacent quaternary ring group minimizes epimerization of this stereocenter.
Oxidation by the active dioxirane catalyst takes place from the si-face, due to steric hindrance of the opposing re-face.
[5] Under normal pH conditions, an excess of 3 stoichiometric amounts of ketone catalyst are needed due to a high rate of decomposition.
The generation of intermediate species number 3 occurs under basic conditions, with a removal of the hydrogen from the hydroxy group to form a nucleophilic oxygen anion.
The activated dioxirane catalytic species then transfers an oxygen atom to the alkene, leading to a regeneration of the original catalyst.
The extent of this side reaction declines with the rise of pH, and increases the nucleophilicity of the oxone, making basic conditions favorable for the overall epoxidation and reactivity of the catalytic species.