Stetter reaction
Unlike 1,3-dicarbonyls, which are easily accessed through the Claisen condensation, or 1,5-dicarbonyls, which are commonly made using a Michael reaction, 1,4-dicarbonyls are challenging substrates to synthesize, yet are valuable starting materials for several organic transformations, including the Paal–Knorr synthesis of furans and pyrroles.
Intramolecular asymmetric Stetter reactions enjoy a range of acceptable Michael acceptors and acyl anion precursors in essentially any combination.
[5] Intramolecular asymmetric Stetter reactions can utilize aromatic, heteroaromatic and aliphatic aldehydes with a tethered α,β-unsaturated ester, ketone, thioester, malonate, nitrile or Weinreb amide.
It has been shown that α,β-unsaturated nitros and aldehydes are not suitable Michael acceptors and have markedly decreased enantiomeric excess in such reactions.
[5] On the other hand, intermolecular asymmetric reactions are quite confined to specifically matched combinations of acyl anion precursor and Michael acceptor, such as an aliphatic aldehyde with a nitroalkene.
In 2001, Murry et al reported a Stetter reaction of aromatic aldehydes onto acylimine derivatives to give α-amido ketone products.
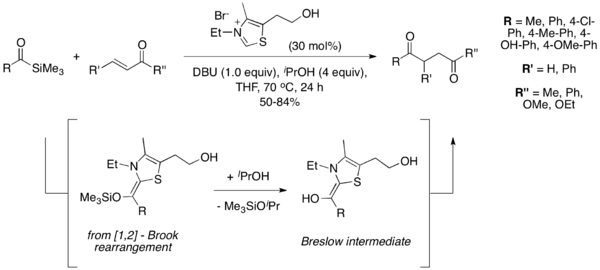
[9] Under the conditions they developed, 2,3-butadienone is cleaved after addition to the thiazolium catalyst to release ethyl acetate and generate the Breslow intermediate necessary for the Stetter reaction to proceed.
In addition, they showed the atom economy and utility of using a cyclic α-diketone to generate the Stetter product with a tethered ethyl ester.
The reaction precedes through the same mechanism as the acyclic version, but the ester generated by attack of ethanol remains tethered to the product.
In 2004, they reported the enantioselective formation of quaternary centers from aromatic aldehydes in an intramolecular Stetter reaction with a slightly modified catalyst.
The Enders group utilized a triazolium-based catalyst to effect the coupling of aromatic aldehydes with chalcone derivatives with moderate yields.
[20] The concurrent publication from the Rovis group also employed a triazolium-based catalyst and reported the Stetter reaction between glyoxamides and alkylidenemalonates in good to excellent yields.
For example, Trost and coworkers employed a Stetter reaction as one step in their synthesis of rac-hirsutic acid C.[24] The intramolecular coupling of an aliphatic aldehyde with a tethered α,β-unsaturated ester led to the desired tricyclic 1,4-dicarbonyl in 67% yield.
The Stetter reaction is commonly used in sequence with the Paal-Knorr synthesis of furans and pyrroles, which a 1,4-dicarbonyl undergoes condensation with itself or in the presence of an amine under high temperature, acidic conditions.
In 2001, Tius and coworkers reported the asymmetric total synthesis of roseophilin utilizing an intermolecular Stetter reaction to couple an aliphatic aldehyde with a cyclic enone.
[26] This procedure first utilizes palladium cross-coupling chemistry to couple aryl halides with propargylic alcohols to give α,β-unsaturated ketones, which can then undergo a Stetter reaction with an aldehyde.