Structure of liquids and glasses
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials.
Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions.
The study of liquid and glass structure aims to gain insight into their behavior and physical properties, so that they can be understood, predicted and tailored for specific applications.
Since the structure and resulting behavior of liquids and glasses is a complex many body problem, historically it has been too computationally intensive to solve using quantum mechanics directly.
Instead, a variety of diffraction, nuclear magnetic resonance (NMR), molecular dynamics, and Monte Carlo simulation techniques are most commonly used.
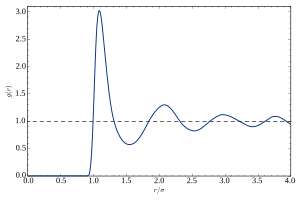
A typical plot of g versus r of a liquid or glass shows a number of key features: The static structure factor, S(q), which can be measured with diffraction techniques, is related to its corresponding g(r) by Fourier transformation where q is the magnitude of the momentum transfer vector, and ρ is the number density of the material.
The g(r) pattern obtained from a diffraction measurement represents a spatial, and thermal average of all the pair correlations in the material, weighted by their coherent cross-sections with the incident beam.
By definition, g(r) is related to the average number of particles found within a given volume of shell located at a distance r from the center.
[6] The tetrahedra in silica also form a network of ring structures which leads to ordering on more intermediate length scales of up to approximately 10 angstroms.
[17] The modifiers (calcium, lead, lithium, sodium, potassium) alter the network structure; they are usually present as ions, compensated by nearby non-bridging oxygen atoms, bound by one covalent bond to the glass network and holding one negative charge to compensate for the positive ion nearby.
[17] The alkali metal ions are small and mobile; their presence in a glass allows a degree of electrical conductivity.
The most common commercial glass types contain both alkali and alkaline earth ions (usually sodium and calcium), for easier processing and satisfying corrosion resistance.
Silica (the chemical compound SiO2) has a number of distinct crystalline forms: quartz, tridymite, cristobalite, and others (including the high pressure polymorphs stishovite and coesite).