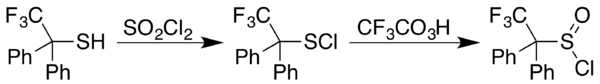
Sulfinyl halide
The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates.
Methanesulfinyl chloride, CH3S(O)Cl, is prepared by chlorination of dimethyl disulfide to give CH3SCl3, which is treated with acetic anhydride.
Treatment of alkanesulfinyl chlorides having α-hydrogens with tertiary amine bases gives thiocarbonyl S-oxides (sulfines) as isolable compounds.
[6] Treatment of methanesulfinyl chloride or ethane-1,2-bis-sulfinyl chloride, ClS(O)CH2CH2S(O)Cl (prepared by oxidative chlorination of 1,2-ethanedithiol, HSCH2CH2SH), with a tertiary amine in the presence of the chiral glucose-derived secondary alcohol diacetone-D-glucose affords optically pure sulfinate esters by a process of Dynamic kinetic resolution.
Room temperature hydrolysis of CF3SF3 gives the sulfinyl fluoride CF3S(O)F in a few hours in quantitative yield.