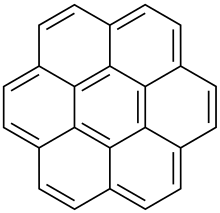
Coronene
Coronene (also known as superbenzene and cyclobenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings.
[12] The presence of coronene putatively formed from contact of magma with fossil fuel deposits has been used to argue that Permian-Triassic “Great Dying” event was caused by a greenhouse gas warming episode triggered by large-scale Siberian vulcanism.
[13] Coronene is produced in the petroleum-refining process of hydrocracking, where it can dimerize to a fifteen ring PAH, trivially named "dicoronylene" .
1 Tesla)[8] or by phase transition from γ decreasing the temperature below 158 K.[14] The structure containing two C-H groups on one benzene ring, so-called DUO, was analyzed by infrared spectroscopy.
For example, coronene molecules evaporated onto a copper surface at 1000 degrees Celsius will form a graphene lattice which can then be transferred onto another substrate.