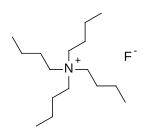
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride, commonly abbreviated to TBAF and n-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−.
[1] Preparing anhydrous samples is of interest as the basicity of fluoride increases by more than 20 pK units on passing from aqueous to aprotic solvent.
[citation needed] However, heating samples of the hydrated material to 77 °C under vacuum causes decomposition to the hydrogen difluoride salt.
[4] Because the fluoride ion is such a strong hydrogen bond acceptor, its salts tend to be hydrated and of limited solubility in organic solvents.
As a fluoride source in organic solvents, TBAF is used to remove silyl ether protecting groups.