Transition metal carbene complex
They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
[5] Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents.
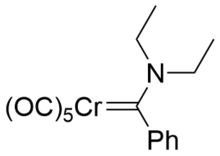
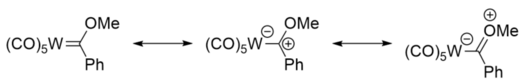
The major resonance structures of Fisher carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
Since there is no donation to the carbene atom from adjacent groups, the extent of pi backbonding is much greater, giving a strong double bond.
Furthermore, the major resonance structures of Schrock carbene put the negative charge on the carbon atom, making it nucleophilic.
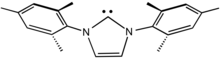
[12][13] Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak.
Like phosphines, NHCs serve as spectator ligands that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates.
[17] Metal carbene complexes have applications in hetereogeneous and homogeneous catalysis, and as reagents for organic reactions.
[4] Homogeneous Schrock-type carbene complexes such as Tebbe's reagent can be used for the olefination of carbonyls, replacing the oxygen atom with a methylidene group.
[6] Diazo compounds like methyl phenyldiazoacetate can be used for cyclopropanation or to insert into C-H bonds of organic substrates.
[9] In 1991, Anthony J. Arduengo synthesized and crystallized the first persistent carbene, an NHC with large adamantane alkyl groups, accelerating the field of N-heterocarbene ligands to its current use.